Pesticides are used extensively on a global scale to protect crops, ensuring they can be successfully grown, stored, and transported to meet consumer demands. The type of pesticide used varies widely depending on the produce in question, with insecticides, herbicides, rodenticides, and fungicides being the most common. A recent review by the Pesticide Action Network showed that there are more than 17,000 pesticide products currently on the market.
Solvent-based pesticides have traditionally been the pesticide of choice, but in light of growing health concerns, less toxic ionic pesticides are being more widely adopted. For example, glyphosate—an anionic pesticide—is now the most widely used pesticide in the world on GMO-engineered glyphosate-resistant crops. Recently, though, there has been growing public concern that any pesticide contamination in food could be a potential health risk, especially as pesticides can often remain in food at trace levels. This has resulted in increased attention from regulatory agencies and health researchers, who are seeking to better understand and monitor these residues.
To ensure that only minimal levels of pesticides are present in food, accurate quantification is required. Many methods exist for determination of pesticides, but gas chromatography (GC) and liquid chromatography (LC) combined with mass spectrometry (MS) are the standard techniques in regulatory test methods; however, these traditional analytical methods aren’t as effective for determining ionic pesticides, as the compounds are too polar to be retained and separated. In addition, it is difficult to maintain low baselines when analyzing ionic pesticides, making them an analytical headache. These challenges in current analytical approaches have been driving the need for more effective analytical techniques to continue protecting public health.
Taking Charge with IC-MS/MS
Ion chromatography coupled with tandem mass spectrometry (IC-MS/MS) can be used to effectively overcome the challenges faced by existing methods when it comes to anionic pesticide determination (see figure 1). Crucially, the technique is ideal for separating polar compounds and has been used to determine anionic polar pesticides such as glyphosate and glufosinate.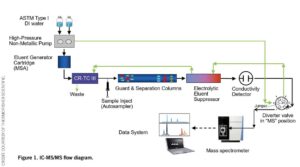
IC-MS/MS has a number of benefits that make it ideally suited for this application. The technique offers high selectivity and sensitivity, as tandem MS detection using selected reaction monitoring (SRM) eliminates sample matrix interference by only scanning for ions of interest. The method also provides low chemical noise, overcoming the baseline issue of GC-MS and LC-MS. With this technique, analytes are also provided in their ionic form, meaning electrospray can be used and the molecular ion retained. Further improvements in pesticide determination are enabled by the electrolytic suppressor, which neutralizes eluent and lowers the background while offering increased sensitivity for conductivity detection and improving the compatibility for MS.
Anionic samples are typically prepared for IC-MS/MS using the quick polar pesticides method (QuPPE) developed by the European Union Reference Laboratory for Pesticide Residues in Fruits and Vegetables (EURL-FV). This acidified methanol-based extraction method has been widely used and accepted for extraction of polar pesticides, according to a 2012 review published in the journal Analytical and Bioanalytical Chemistry, giving excellent results. IC-MS/MS used with QuPPE extraction provides a highly useful and sensitive approach for anionic pesticide determination, ultimately helping analytical scientists to better protect public health.
The Rise of Cationic Pesticides
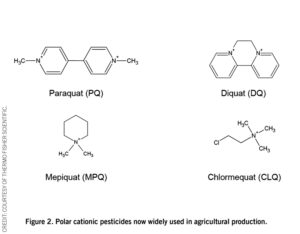
Cationic quaternary amines, or quats, are a new class of ionic pesticide now gaining popularity. Unlike glyphosate, quats are permanently charged species, regardless of pH. Of these, paraquat, diquat, mepiquat, and chlormequat (see figure 2) are among the most important and commonly used.
Although ionic pesticides are generally less toxic than solvent-based ones, compounds such as paraquat and diquat are still highly toxic. Often, these pesticides are used late in the plant’s life as desiccants to kill the plant before the harvest. By doing this, farmers can bring the crops in earlier, before they are contaminated with mold during the rainy season. While this practice helps to guarantee the food supply, the late addition of these pesticides to the crop can cause problems as they can bind to the plant, creating a higher risk of food supply contamination.
ACCESS THE FULL VERSION OF THIS ARTICLE
To view this article and gain unlimited access to premium content on the FQ&S website, register for your FREE account. Build your profile and create a personalized experience today! Sign up is easy!
GET STARTED
Already have an account? LOGIN