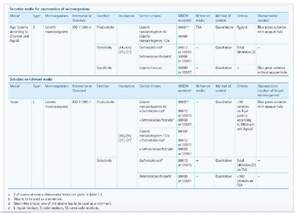
For laboratories that continue to produce their culture media in-house, each batch can sufficiently be tested using a single test strain named in the standard. Manufacturers, on the other hand, must test each batch using several microorganisms. Laboratories can rely upon the performance tests that the manufacturer conducts for ready-to-use media only as long as the transport conditions are observed and the manufacturer’s QC test is performed according to the requirements of the standard. It is the end users responsibility to ensure that batch testing was performed according the requirements of EN ISO 11133:2014 by procuring the quality control certificate as a supporting document from the manufacturer. This certificate should disclose the test organisms used, the acceptance criteria of the performance tests, and the test results.
Performance Testing
Suppliers must conduct rigorous qualitative and/or quantitative testing on all ISO 11133:2014 compliant culture media that they provide to laboratories. Below are a few examples of testing criteria for liquid and solid selective and non-selective culture media.
Liquid Culture Media. Buffered Peptone Water (BPW) is used for the non-selective pre-enrichment of Salmonella bacteria in food. The EN ISO 11133:2014 testing criterion for BPW is turbidity, which must either be weak or good.
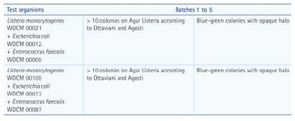
Fraser broth is used for the selective enrichment of Listeria monocytogenes in food. The EN ISO 11133:2014 testing criterion for Fraser broth is the growth of more than 10 colonies on Listeria selective agar according to Ottaviani and Agosti. When inoculated as a mixed culture with Escherichia coli and Enterococcus faecalis, only L. monocytogenes should be able to grow on this agar as characteristic blue-green colonies with opaque halos.
Table 2 at right shows the results of qualitative tests performed for five batches of Fraser broth (EMD Millipore). The E. coli and E. faecalis strains are included in the testing criterion to prove the selective properties of the medium.
Solid Agar Media. Two common solid agar media from EMD Millipore are selective de Man, Rogosa, Sharpe (MRS) agar for enumeration of lactic acid, and non-selective Plate Count Agar (PCA) for colony count. The EN ISO 11133:2014 quantitative productivity testing criterion for each of these solid media agar is recovery rates of 70 percent or more for every test organism. Figure 1 on page 41 shows the results of quantitative tests performed for five batches of MRS and PCA medium.
Conclusions
EN ISO 11133:2014 is a mandatory standard for all accredited laboratories that perform microbiological testing of food, animal feed, or water using culture media. The goal of this new standard is to improve consumer safety with respect to food and beverage products, and the guiding principle is that performance testing conditions should mimic the intended sample testing conditions as closely as possible.
Many laboratories source their culture media from suppliers both to streamline their workflows and to ensure high quality and batch-to-batch consistency.
Under the new standard, laboratories that source their culture media from a supplier can ensure that the media is manufactured and certified according to the latest international standard, EN ISO 11133:2014, by procuring the quality control certificate as a supporting document. Ultimately, this standard should reduce the workload for the qualification of new culture media batches procured from suppliers. In the supporting document, suppliers should provide quantitative information about the growth of both “wanted” microorganisms (bacteria that should grow on a specific medium) and “unwanted” microorganisms (bacteria that should not grow on a specific medium). The highest quality media will support only the growth of “wanted” microorganisms.
ACCESS THE FULL VERSION OF THIS ARTICLE
To view this article and gain unlimited access to premium content on the FQ&S website, register for your FREE account. Build your profile and create a personalized experience today! Sign up is easy!
GET STARTED
Already have an account? LOGIN